|

by
John Gribbin
from
SkyBooksUSA
Website
Contents
Quantum
 Quantum Theory, also quantum mechanics, in physics, a theory based
on using the concept of the quantum unit to describe the dynamic
properties of subatomic particles and the interactions of matter and
radiation. The foundation was laid by the German physicist Max
Planck, who postulated in 1900 that energy can be emitted or
absorbed by matter only in small, discrete units called quanta. Quantum Theory, also quantum mechanics, in physics, a theory based
on using the concept of the quantum unit to describe the dynamic
properties of subatomic particles and the interactions of matter and
radiation. The foundation was laid by the German physicist Max
Planck, who postulated in 1900 that energy can be emitted or
absorbed by matter only in small, discrete units called quanta.
Also
fundamental to the development of quantum mechanics was the
uncertainty principle, formulated by the German physicist Werner
Heisenberg in 1927, which states that the position and momentum of a
subatomic particle cannot be specified simultaneously.
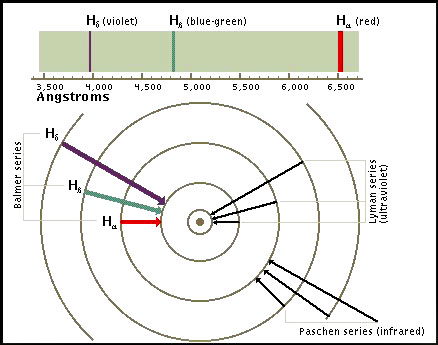
Spectral Lines of Atomic Hydrogen: When an electron makes a
transition from one energy level to another, the electron emits a
photon with a particular energy. These photons are then observed as
emission lines using a spectroscope. The Lyman series involves
transitions to the lowest or ground state energy level.
Transitions
to the second energy level are called the Balmer series. These
transitions involve frequencies in the visible part of the spectrum.
In this frequency range each transition is characterized by a
different color.
Go Back
Early History
In the 18th and 19th centuries, Newtonian, or classical, mechanics
appeared to provide a wholly accurate description of the motions of
bodies—for example, planetary motion. In the late 19th and early
20th centuries, however, experimental findings raised doubts about
the completeness of Newtonian theory. Among the newer observations
were the lines that appear in the spectra of light emitted by heated
gases, or gases in which electric discharges take place.
From the
model of the atom developed in the early 20th century by the English
physicist Ernest Rutherford, in which negatively charged electrons
circle a positive nucleus in orbits prescribed by Newton’s laws of
motion, scientists had also expected that the electrons would emit
light over a broad frequency range, rather than in the narrow
frequency ranges that form the lines in a spectrum.
Another puzzle for physicists was the coexistence of two theories of
light: the corpuscular theory, which explains light as a stream of
particles, and the wave theory, which views light as electromagnetic
waves. A third problem was the absence of a molecular basis for
thermodynamics. In his book Elementary Principles in Statistical
Mechanics (1902), the American mathematical physicist J. Willard
Gibbs conceded the impossibility of framing a theory of molecular
action that reconciled thermodynamics, radiation, and electrical
phenomena as they were then understood.
Go Back
Planck’s Contribution
At the turn of the century, physicists did not yet clearly recognize
that these and other difficulties in physics were in any way
related. The first development that led to the solution of these
difficulties was Planck’s introduction of the concept of the
quantum, as a result of physicists’ studies of blackbody radiation
during the closing years of the 19th century. (The term blackbody
refers to an ideal body or surface that absorbs all radiant energy
without any reflection.)
A body at a moderately high temperature — a
"red heat" — gives off most of its radiation in the low frequency (red
and infrared) regions; a body at a higher temperature — "white
heat" — gives off comparatively more radiation in higher frequencies
(yellow, green, or blue). During the 1890s physicists conducted
detailed quantitative studies of these phenomena and expressed their
results in a series of curves or graphs. The classical, or prequantum, theory predicted an altogether different set of curves
from those actually observed.
What Planck did was to devise a
mathematical formula that described the curves exactly; he then
deduced a physical hypothesis that could explain the formula. His
hypothesis was that energy is radiated only in quanta of energy hu,
where u is the frequency and h is the quantum action, now known as
Planck’s constant.
Go Back
Einstein Contribution
The next important developments in quantum mechanics were the work
of German-born American physicist and Nobel laureate Albert
Einstein. He used Planck’s concept of the quantum to explain certain
properties of the photoelectric effect—an experimentally observed
phenomenon in which electrons are emitted from metal surfaces when
radiation falls on these surfaces.
According to classical theory, the energy, as measured by the
voltage of the emitted electrons, should be proportional to the
intensity of the radiation. The energy of the electrons, however,
was found to be independent of the intensity of radiation—which
determined only the number of electrons emitted—and to depend solely
on the frequency of the radiation. The higher the frequency of the
incident radiation, the greater is the electron energy; below a
certain critical frequency no electrons are emitted. These facts
were explained by Einstein by assuming that a single quantum of
radiant energy ejects a single electron from the metal.
The energy
of the quantum is proportional to the frequency, and so the energy
of the electron depends on the frequency.
Go Back
Bohr Atom
In 1911 Rutherford established the existence of the atomic nucleus.
He assumed, on the basis of experimental evidence obtained from the
scattering of alpha particles by the nuclei of gold atoms, that
every atom consists of a dense, positively charged nucleus,
surrounded by negatively charged electrons revolving around the
nucleus as planets revolve around the sun.
The classical
electromagnetic theory developed by the British physicist James
Clerk Maxwell unequivocally predicted that an electron revolving
around a nucleus will continuously radiate electromagnetic energy
until it has lost all its energy, and eventually will fall into the
nucleus. Thus, according to classical theory, an atom, as described
by Rutherford, is unstable. This difficulty led the Danish physicist
Niels Bohr, in 1913, to postulate that in an atom the classical
theory does not hold, and that electrons move in fixed orbits. Every
change in orbit by the electron corresponds to the absorption or
emission of a quantum of radiation.
The application of Bohr’s theory to atoms with more than one
electron proved difficult. The mathematical equations for the next
simplest atom, the helium atom, were solved during the 1910s and
1920s, but the results were not entirely in accordance with
experiment. For more complex atoms, only approximate solutions of
the equations are possible, and these are only partly concordant
with observations.
Go Back
Wave Mechanics
The French physicist Louis Victor de Broglie suggested in 1924 that
because electromagnetic waves show particle characteristics,
particles should, in some cases, also exhibit wave properties. This
prediction was verified experimentally within a few years by the
American physicists Clinton Joseph Davisson and Lester Halbert
Germer and the British physicist George Paget Thomson. They showed
that a beam of electrons scattered by a crystal produces a
diffraction pattern characteristic of a wave (see Diffraction). The
wave concept of a particle led the Austrian physicist Erwin
Schrödinger to develop a so-called wave equation to describe the
wave properties of a particle and, more specifically, the wave
behavior of the electron in the hydrogen atom.
Although this differential equation was continuous and gave
solutions for all points in space, the permissible solutions of the
equation were restricted by certain conditions expressed by
mathematical equations called eigenfunctions (German eigen, "own").
The Schrödinger wave equation thus had only certain discrete
solutions; these solutions were mathematical expressions in which
quantum numbers appeared as parameters. (Quantum numbers are
integers developed in particle physics to give the magnitudes of
certain characteristic quantities of particles or systems.)
The
Schrödinger equation was solved for the hydrogen atom and gave
conclusions in substantial agreement with earlier quantum theory.
Moreover, it was solvable for the helium atom, which earlier theory
had failed to explain adequately, and here also it was in agreement
with experimental evidence. The solutions of the Schrödinger
equation also indicated that no two electrons could have the same
four quantum numbers—that is, be in the same energy state.
This
rule, which had already been established empirically by
Austro-American physicist and Nobel laureate Wolfgang Pauli in 1925,
is called the exclusion principle.
Go Back
What is Matter
In the 20th century, physicists discovered that matter behaved as
both a wave and a particle. Austrian physicist and Nobel Prize
winner Erwin Schrödinger discussed this apparent paradox in a
lecture in Geneva, Switzerland, in 1952. A condensed and translated
version of his lecture appeared in Scientific American the following
year.
What Is Matter?
The wave-particle dualism afflicting modern physics is best resolved
in favor of waves, believes the author, but there is no clear
picture of matter on which physicists can agree
Fifty years ago science seemed on the road to a clear-cut answer to
the ancient question which is the title of this article. It looked
as if matter would be reduced at last to its ultimate building
blocks—to certain submicroscopic but nevertheless tangible and
measurable particles. But it proved to be less simple than that.
Today a physicist no longer can distinguish significantly between
matter and something else. We no longer contrast matter with forces
or fields of force as different entities; we know now that these
concepts must be merged. It is true that we speak of "empty" space
(i.e., space free of matter), but space is never really empty,
because even in the remotest voids of the universe there is always
starlight—and that is matter. Besides, space is filled with
gravitational fields, and according to Einstein gravity and inertia
cannot very well be separated.
Thus the subject of this article is in fact the total picture of
space-time reality as envisaged by physics. We have to admit that
our conception of material reality today is more wavering and
uncertain than it has been for a long time. We know a great many
interesting details, learn new ones every week. But to construct a
clear, easily comprehensible picture on which all physicists would
agree—that is simply impossible.
Physics stands at a grave crisis of
ideas. In the face of this crisis, many maintain that no objective
picture of reality is possible. However, the optimists among us (of
whom I consider myself one) look upon this view as a philosophical
extravagance born of despair. We hope that the present fluctuations
of thinking are only indications of an upheaval of old beliefs which
in the end will lead to something better than the mess of formulas
which today surrounds our subject.
Since the picture of matter that I am supposed to draw does not yet
exist, since only fragments of it are visible, some parts of this
narrative may be inconsistent with others. Like Cervantes’ tale of Sancho Panza, who loses his donkey in one chapter but a few chapters
later, thanks to the forgetfulness of the author, is riding the dear
little animal again, our story has contradictions. We must start
with the well-established concept that matter is composed of
corpuscles or atoms, whose existence has been quite "tangibly"
demonstrated by many beautiful experiments, and with Max Planck’s
discovery that energy also comes in indivisible units, called
quanta, which are supposed to be transferred abruptly from one
carrier to another.
But then Sancho Panza’s donkey will return. For I shall have to ask
you to believe neither in corpuscles as permanent individuals nor in
the suddenness of the transfer of an energy quantum. Discreteness is
present, but not in the traditional sense of discrete single
particles, let alone in the sense of abrupt processes. Discreteness
arises merely as a structure from the laws governing the phenomena.
These laws are by no means fully understood; a probably correct
analogue from the physics of palpable bodies is the way various
partial tones of a bell derive from its shape and from the laws of
elasticity to which, of themselves, nothing discontinuous adheres.
The idea that matter is made up of ultimate particles was advanced
as early as the fifth century B.C. by Leucippus and Democritus, who
called these particles atoms. The corpuscular theory of matter was
lifted to physical reality in the theory of gases developed during
the 19th century by James Clerk Maxwell and Ludwig Boltzmann. The
concept of atoms and molecules in violent motion, colliding and
rebounding again and again, led to full comprehension of all the
properties of gases: their elastic and thermal properties, their
viscosity, heat conductivity and diffusion. At the same time it led
to a firm foundation of the mechanical theory of heat, namely, that
heat is the motion of these ultimate particles, which becomes
increasingly violent with rising temperature.
Within one tremendously fertile decade at the turn of the century
came the discoveries of X-rays, of electrons, of the emission of
streams of particles and other forms of energy from the atomic
nucleus by radioactive decay, of the electric charges on the various
particles. The masses of these particles, and of the atoms
themselves, were later measured very precisely, and from this was
discovered the mass defect of the atomic nucleus as a whole. The
mass of a nucleus is less than the sum of the masses of its
component particles; the lost mass becomes the binding energy
holding the nucleus firmly together. This is called the packing
effect. The nuclear forces of course are not electrical forces—those
are repellent—but are much stronger and act only within very short
distances, about 10-13 centimeter.
Here I am already caught in a contradiction. Didn’t I say at the
beginning that we no longer assume the existence of force fields
apart from matter? I could easily talk myself out of it by saying:
Well, the force field of a particle is simply considered a part of
it. But that is not the fact. The established view today is rather
that everything is at the same time both particle and field.
Everything has the continuous structure with which we are familiar
in fields, as well as the discrete structure with which we are
equally familiar in particles. This concept is supported by
innumerable experimental facts and is accepted in general, though
opinions differ on details, as we shall see.
In the particular case of the field of nuclear forces, the particle
structure is more or less known. Most likely the continuous force
field is represented by the so-called pi mesons. On the other hand,
the protons and neutrons, which we think of as discrete particles,
indisputably also have a continuous wave structure, as is shown by
the interference patterns they form when diffracted by a crystal.
The difficulty of combining these two so very different character
traits in one mental picture is the main stumbling-block that causes
our conception of matter to be so uncertain.
Neither the particle concept nor the wave concept is hypothetical.
The tracks in a photographic emulsion or in a Wilson cloud chamber
leave no doubt of the behavior of particles as discrete units. The
artificial production of nuclear particles is being attempted right
now with terrific expenditure, defrayed in the main by the various
state ministries of defense. It is true that one cannot kill anybody
with one such racing particle, or else we should all be dead by now.
But their study promises, indirectly, a hastened realization of the
plan for the annihilation of mankind which is so close to all our
hearts.
You can easily observe particles yourself by looking at a luminous
numeral of your wrist watch in the dark with a magnifying glass. The
luminosity surges and undulates, just as a lake sometimes twinkles
in the sun. The light consists of sparklets, each produced by a
so-called alpha particle (helium nucleus) expelled by a radioactive
atom which in this process is transformed into a different atom. A
specific device for detecting and recording single particles is the
Geiger-Müller counter. In this short résumé I cannot possibly
exhaust the many ways in which we can observe single particles.
Now to the continuous field or wave character of matter. Wave
structure is studied mainly by means of diffraction and
interference—phenomena which occur when wave trains cross each
other. For the analysis and measurement of light waves the principal
device is the ruled grating, which consists of a great many fine,
parallel, equidistant lines, closely engraved on a specular metallic
surface.
Light impinging from one direction is scattered by them and
collected in different directions depending on its wavelength. But
even the finest ruled gratings we can produce are too coarse to
scatter the very much shorter waves associated with matter. The fine
lattices of crystals, however, which Max von Laue first used as
gratings to analyze the very short X-rays, will do the same for
"matter waves." Directed at the surface of a crystal, high-velocity
streams of particles manifest their wave nature. With crystal
gratings physicists have diffracted and measured the wavelengths of
electrons, neutrons and protons.
What does Planck’s quantum theory have to do with all this? Planck
told us in 1900 that he could comprehend the radiation from red-hot
iron, or from an incandescent star such as the sun, only if this
radiation was produced in discrete portions and transferred in such
discrete quantities from one carrier to another (e.g., from atom to
atom).
This was extremely startling, because up to that time energy
had been a highly abstract concept. Five years later Einstein told
us that energy has mass and mass is energy; in other words, that
they are one and the same. Now the scales begin to fall from our
eyes: our dear old atoms, corpuscles, particles are Planck’s energy
quanta. The carriers of those quanta are themselves quanta. One gets
dizzy. Something quite fundamental must lie at the bottom of this,
but it is not surprising that the secret is not yet understood.
After all, the scales did not fall suddenly. It took 20 or 30 years.
And perhaps they still have not fallen completely.
The next step was not quite so far reaching, but important enough.
By an ingenious and appropriate generalization of Planck’s
hypothesis Niels Bohr taught us to understand the line spectra of
atoms and molecules and how atoms were composed of heavy, positively
charged nuclei with light, negatively charged electrons revolving
around them. Each small system—atom or molecule—can harbor only
definite discrete energy quantities, corresponding to its nature or
its constitution. In transition from a higher to a lower "energy
level" it emits the excess energy as a radiation quantum of definite
wavelength, inversely proportional to the quantum given off. This
means that a quantum of given magnitude manifests itself in a
periodic process of definite frequency which is directly
proportional to the quantum; the frequency equals the energy quantum
divided by the famous Planck’s constant, h.
According to Einstein a particle has the energy mc2, m being the
mass of the particle and c the velocity of light. In 1925 Louis de Broglie drew the inference, which rather suggests itself, that a
particle might have associated with it a wave process of frequency
mc2 divided by h. The particle for which he postulated such a wave
was the electron. Within two years the "electron waves" required by
his theory were demonstrated by the famous electron diffraction
experiment of C. J. Davisson and L. H. Germer.
This was the starting
point for the cognition that everything — anything at all — is
simultaneously particle and wave field. Thus de Broglie’s
dissertation initiated our uncertainty about the nature of matter.
Both the particle picture and the wave picture have truth value, and
we cannot give up either one or the other. But we do not know how to
combine them.
That the two pictures are connected is known in full generality with
great precision and down to amazing details. But concerning the
unification to a single, concrete, palpable picture opinions are so
strongly divided that a great many deem it altogether impossible. I
shall briefly sketch the connection. But do not expect that a
uniform, concrete picture will emerge before you; and do not blame
the lack of success either on my ineptness in exposition or your own
denseness—nobody has yet succeeded.
One distinguishes two things in a wave. First of all, a wave has a
front, and a succession of wave fronts forms a system of surfaces
like the layers of an onion. You are familiar with the
two-dimensional analogue of the beautiful wave circles that form on
the smooth surface of a pond when a stone is thrown in. The second
characteristic of a wave, less intuitive, is the path along which it
travels—a system of imagined lines perpendicular to the wave fronts.
These lines are known as the wave "normals" or "rays."
We can make the provisional assertion that these rays correspond to
the trajectories of particles. Indeed, if you cut a small piece out
of a wave, approximately 10 or 20 wavelengths along the direction of
propagation and about as much across, such a "wave packet" would
actually move along a ray with exactly the same velocity and change
of velocity as we might expect from a particle of this particular
kind at this particular place, taking into account any force fields
acting on the particle.
Here I falter. For what I must say now, though correct, almost
contradicts this provisional assertion. Although the behavior of the
wave packet gives us a more or less intuitive picture of a particle,
which can be worked out in detail (e.g., the momentum of a particle
increases as the wavelength decreases; the two are inversely
proportional), yet for many reasons we cannot take this intuitive
picture quite seriously. For one thing, it is, after all, somewhat
vague, the more so the greater the wavelength. For another, quite
often we are dealing not with a small packet but with an extended
wave. For still another, we must also deal with the important
special case of very small "packelets" which form a kind of
"standing wave" which can have no wave fronts or wave normals.
One interpretation of wave phenomena which is extensively supported
by experiments is this: At each position of a uniformly propagating
wave train there is a twofold structural connection of interactions,
which may be distinguished as "longitudinal" and "transversal." The
transversal structure is that of the wave fronts and manifests
itself in diffraction and interference experiments; the longitudinal
structure is that of the wave normals and manifests itself in the
observation of single particles. However, these concepts of
longitudinal and transversal structures are not sharply defined and
absolute, since the concepts of wave front and wave normal are not,
either.
The interpretation breaks down completely in the special case of the
standing waves mentioned above. Here the whole wave phenomenon is
reduced to a small region of the dimensions of a single or very few
wavelengths. You can produce standing water waves of a similar
nature in a small basin if you dabble with your finger rather
uniformly in its center, or else just give it a little push so that
the water surface undulates. In this situation we are not dealing
with uniform wave propagation; what catches the interest are the
normal frequencies of these standing waves.
The water waves in the
basin are an analogue of a wave phenomenon associated with
electrons, which occurs in a region just about the size of the atom.
The normal frequencies of the wave group washing around the atomic
nucleus are universally found to be exactly equal to Bohr’s atomic
"energy levels" divided by Planck’s constant h. Thus the ingenious
yet somewhat artificial assumptions of Bohr’s model of the atom, as
well as of the older quantum theory in general, are superseded by
the far more natural idea of de Broglie’s wave phenomenon.
The wave
phenomenon forms the "body" proper of the atom. It takes the place
of the individual pointlike electrons which in Bohr’s model are
supposed to swarm around the nucleus. Such pointlike single
particles are completely out of the question within the atom, and if
one still thinks of the nucleus itself in this way one does so quite
consciously for reasons of expediency.
What seems to me particularly important about the discovery that
"energy levels" are virtually nothing but the frequencies of normal
modes of vibration is that now one can do without the assumption of
sudden transitions, or quantum jumps, since two or more normal modes
may very well be excited simultaneously. The discreteness of the
normal frequencies fully suffices—so I believe—to support the
considerations from which Planck started and many similar and just
as important ones—I mean, in short, to support all of quantum
thermodynamics.
The theory of quantum jumps is becoming more and more unacceptable,
at least to me personally, as the years go on. Its abandonment has,
however, far-reaching consequences. It means that one must give up
entirely the idea of the exchange of energy in well-defined quanta
and replace it with the concept of resonance between vibrational
frequencies. Yet we have seen that because of the identity of mass
and energy, we must consider the particles themselves as Planck’s
energy quanta. This is at first frightening. For the substituted
theory implies that we can no longer consider the individual
particle as a well-defined permanent entity.
That it is, in fact, no such thing can be reasoned in other ways.
For one thing, there is Werner Heisenberg’s famous uncertainty
principle, according to which a particle cannot simultaneously have
a well-defined position and a sharply defined velocity. This
uncertainty implies that we cannot be sure that the same particle
could ever be observed twice.
Another conclusive reason for not
attributing identifiable sameness to individual particles is that we
must obliterate their individualities whenever we consider two or
more interacting particles of the same kind, e.g., the two electrons
of a helium atom. Two situations which are distinguished only by the
interchange of the two electrons must be counted as one and the
same; if they are counted as two equal situations, nonsense obtains.
This circumstance holds for any kind of particle in arbitrary
numbers without exception.
Most theoreticians will probably accept the foregoing reasoning and
admit that the individual particle is not a well-defined permanent
entity of detectable identity or sameness. Nevertheless this
inadmissible concept of the individual particle continues to play a
large role in their ideas and discussions. Even deeper rooted is the
belief in "quantum jumps," which is now surrounded with a highly
abstruse terminology whose common-sense meaning is often difficult
to grasp.
For instance, an important word in the standing vocabulary
of quantum theory is "probability," referring to transition from one
level to another. But, after all, one can speak of the probability
of an event only assuming that, occasionally, it actually occurs. If
it does occur, the transition must indeed be sudden, since
intermediate stages are disclaimed. Moreover, if it takes time, it
might conceivably be interrupted halfway by an unforeseen
disturbance. This possibility leaves one completely at sea.
The wave v. corpuscle dilemma is supposed to be resolved by
asserting that the wave field merely serves for the computation of
the probability of finding a particle of given properties at a given
position if one looks for it there. But once one deprives the waves
of reality and assigns them only a kind of informative role, it
becomes very difficult to understand the phenomena of interference
and diffraction on the basis of the combined action of discrete
single particles. It certainly seems easier to explain particle
tracks in terms of waves than to explain the wave phenomenon in
terms of corpuscles.
"Real existence" is, to be sure, an expression which has been
virtually chased to death by many philosophical hounds. Its simple,
naive meaning has almost become lost to us. Therefore I want to
recall something else. I spoke of a corpuscle’s not being an
individual. Properly speaking, one never observes the same particle
a second time—very much as Heraclitus says of the river. You cannot
mark an electron, you cannot paint it red. Indeed, you must not even
think of it as marked; if you do, your "counting" will be false and
you will get wrong results at every step—for the structure of line
spectra, in thermodynamics and elsewhere. A wave, on the other hand,
can easily be imprinted with an individual structure by which it can
be recognized beyond doubt. Think of the beacon fires that guide
ships at sea.
The light shines according to a definite code; for
example: three seconds light, five seconds dark, one second light,
another pause of five seconds, and again light for three seconds—the
skipper knows that is San Sebastian. Or you talk by wireless
telephone with a friend across the Atlantic; as soon as he says,
"Hello there, Edward Meier speaking," you know that his voice has
imprinted on the radio wave a structure which can be distinguished
from any other.
But one does not have to go that far. If your wife
calls, "Francis!" from the garden, it is exactly the same thing,
except that the structure is printed on sound waves and the trip is
shorter (though it takes somewhat longer than the journey of radio
waves across the Atlantic). All our verbal communication is based on
imprinted individual wave structures. And, according to the same
principle, what a wealth of details is transmitted to us in rapid
succession by the movie or the television picture!
This characteristic, the individuality of the wave phenomenon, has
already been found to a remarkable extent in the very much finer
waves of particles. One example must suffice. A limited volume of
gas, say helium, can be thought of either as a collection of many
helium atoms or as a superposition of elementary wave trains of
matter waves. Both views lead to the same theoretical results as to
the behavior of the gas upon heating, compression, and so on.
But
when you attempt to apply certain somewhat involved enumerations to
the gas, you must carry them out in different ways according to the
mental picture with which you approach it. If you treat the gas as
consisting of particles, then no individuality must be ascribed to
them, as I said. If, however, you concentrate on the matter wave
trains instead of on the particles, every one of the wave trains has
a well-defined structure which is different from that of any other.
It is true that there are many pairs of waves which are so similar
to each other that they could change roles without any noticeable
effect on the gas. But if you should count the very many similar
states formed in this way as merely a single one, the result would
be quite wrong.
In spite of everything we cannot completely banish the concepts of
quantum jump and individual corpuscle from the vocabulary of
physics. We still require them to describe many details of the
structure of matter. How can one ever determine the weight of a
carbon nucleus and of a hydrogen nucleus, each to the precision of
several decimals, and detect that the former is somewhat lighter
than the 12 hydrogen nuclei combined in it, without accepting for
the time being the view that these particles are something quite
concrete and real?
This view is so much more convenient than the
roundabout consideration of wave trains that we cannot do without
it, just as the chemist does not discard his valence-bond formulas,
although he fully realizes that they represent a drastic
simplification of a rather involved wave-mechanical situation.
If you finally ask me: "Well, what are these corpuscles, really?" I
ought to confess honestly that I am almost as little prepared to
answer that as to tell where Sancho Panza’s second donkey came from.
At the most, it may be permissible to say that one can think of
particles as more or less temporary entities within the wave field
whose form and general behavior are nevertheless so clearly and
sharply determined by the laws of waves that many processes take
place as if these temporary entities were substantial permanent
beings. The mass and the charge of particles, defined with such
precision, must then be counted among the structural elements
determined by the wave laws.
The conservation of charge and mass in
the large must be considered as a statistical effect, based on the
"law of large numbers."
Go Back
Matrix Mechanics
Simultaneously with the development of wave mechanics, Heisenberg
evolved a different mathematical analysis known as matrix mechanics.
According to Heisenberg’s theory, which was developed in
collaboration with the German physicists Max Born and Ernst Pascual
Jordan, the formula was not a differential equation but a matrix: an
array consisting of an infinite number of rows, each row consisting
of an infinite number of quantities.
Matrix mechanics introduced
infinite matrices to represent the position and momentum of an
electron inside an atom. Also, different matrices exist, one for
each observable physical property associated with the motion of an
electron, such as energy, position, momentum, and angular momentum.
These matrices, like Schrödinger’s differential equations, could be
solved; in other words, they could be manipulated to produce
predictions as to the frequencies of the lines in the hydrogen
spectrum and other observable quantities.
Like wave mechanics,
matrix mechanics was in agreement with the earlier quantum theory
for processes in which the earlier quantum theory agreed with
experiment; it was also useful in explaining phenomena that earlier
quantum theory could not explain.
Go Back
Quantum Meaning
Schrödinger subsequently succeeded in showing that wave mechanics
and matrix mechanics are different mathematical versions of the same
theory, now called quantum mechanics. Even for the simple hydrogen
atom, which consists of two particles, both mathematical
interpretations are extremely complex. The next simplest atom,
helium, has three particles, and even in the relatively simple
mathematics of classical dynamics, the three-body problem (that of
describing the mutual interactions of three separate bodies) is not
entirely soluble.
The energy levels can be calculated accurately,
however, even if not exactly. In applying quantum-mechanics
mathematics to relatively complex situations, a physicist can use
one of a number of mathematical formulations. The choice depends on
the convenience of the formulation for obtaining suitable
approximate solutions.
Although quantum mechanics describes the atom purely in terms of
mathematical interpretations of observed phenomena, a rough verbal
description can be given of what the atom is now thought to be like.
Surrounding the nucleus is a series of stationary waves; these waves
have crests at certain points, each complete standing wave
representing an orbit. The absolute square of the amplitude of the
wave at any point is a measure of the probability that an electron
will be found at that point at any given time.
Thus, an electron can
no longer be said to be at any precise point at any given time.
Go Back
Uncertainties
The impossibility of pinpointing an electron at any precise time was
analyzed by Heisenberg, who in 1927 formulated the uncertainty
principle. This principle states the impossibility of simultaneously
specifying the precise position and momentum of any particle. In
other words, the more accurately a particle’s momentum is measured
and known, the less accuracy there can be in the measurement and
knowledge of its position. This principle is also fundamental to the
understanding of quantum mechanics as it is generally accepted
today: The wave and particle character of electromagnetic radiation
can be understood as two complementary properties of radiation.
Another way of expressing the uncertainty principle is that the
wavelength of a quantum mechanical principle is inversely
proportional to its momentum. As atoms are cooled they slow down and
their corresponding wavelength grows larger. At a low enough
temperature this wavelength is predicted to exceed the spacing
between particles, causing atoms to overlap, becoming
indistinguishable, and melding into a single quantum state. In 1995
a team of Colorado scientists, led by National Institutes of
Standards and Technology physicist Eric Cornell and University of
Colorado physicist Carl Weiman, cooled rubidium atoms to a
temperature so low that the particles entered this merged state,
known as a Bose-Einstein condensate.
The condensate essentially
behaves like one atom even though it is made up of thousands.
Go Back
Bose-Einstein
- Physicists Condense Supercooled Atoms, Forming New State of Matter
A team of Colorado physicists has cooled atoms of gas to a
temperature so low that the particles entered a merged state, known
as a "Bose-Einstein condensate." This phenomenon was first predicted
about 70 years ago by the theories of German-born American physicist
Albert Einstein and Indian physicist Satyendra Nath Bose. The
condensed particles are considered a new state of matter, different
from the common states of matter—gas, liquid, and solid—and from
plasma, a high temperature, ionized form of matter that is found in
the sun and other stars.
Physicists have great expectations for the application of this
discovery. Because the condensate essentially behaves like one atom
even though it is made up of thousands, investigators should be able
to measure interactions at the atomic and subatomic level that were
previously extremely difficult, if not impossible, to study
quantitatively.
The condensate was detected June 5 by a Colorado team led by
National Institutes of Standards and Technology physicist Eric
Cornell and University of Colorado physicist Carl Wieman. Their
discovery was reported in the journal Science on July 14. Cornell
and Wieman formed their condensate from rubidium gas.
Several groups of physicists, including the teams in Texas and
Colorado and a group at the Massachusetts Institute of Technology,
have been working to form pure condensate in recent years. The goal
of the investigations has been to create a pure chunk of condensate
out of atoms in an inert medium, such as a diffuse, nonreactive gas.
The effort began when methods of cooling and trapping became refined
enough that it seemed possible to reach the required conditions of
temperature and density.
The Colorado team used two techniques: first laser cooling and then
evaporative cooling. The laser technique used laser light whose
frequency was carefully tuned to interact with the rubidium atoms
and gently reduce their speeds. A number of lasers were aimed at the
gas to slow the motion of the atoms in different directions.
The Colorado physicists then switched to evaporative cooling. In
this method, the gas is "trapped" by a magnetic field that dwindles
to zero at its center. Atoms that are moving wander out of the
field, while the coldest atoms cluster at the center. Because a few
very cold atoms could still escape at the zero field point of the
trap, the physicists perfected their system by adding a second
slowly circling magnetic field so that the zero point moved, not
giving the atoms the chance to escape through it.
Physicists will now begin to explore the properties of the
condensate and see what other materials they can use to form it. One
unusual characteristic of the condensate is that it is composed of
atoms that have lost their individual identities. This is analogous
to laser light, which is composed of light particles, or photons,
that similarly have become indistinguishable and all behave in
exactly the same manner. The laser has found a myriad of uses both
in practical applications and in theoretical research, and the
Bose-Einstein condensate may turn out to be just as important. Some
scientists speculate that if a condensate can be readily produced
and sustained, it could be used to miniaturize and speed up computer
components to a scale and quickness not possible before.
The prediction that a merged form of matter will emerge at extremely
low temperatures is based on a number of aspects of the quantum
theory. This theory governs the interaction of particles on a
subatomic scale. The basic principle of quantum theory is that
particles can only exist in certain discrete energy states.
The exact "quantum state" of a particle takes into consideration
such factors as the position of the particle and its "spin," which
can only have certain discrete values. A particle’s spin categorizes
it as either a boson or a fermion. Those two groups of particles
behave according to different sets of statistical rules. Bosons have
spins that are a constant number multiplied by an integer (e.g., 0,
1, 2, 3). Fermions have spins that are that same constant multiplied
by an odd half-integer (1/2, 3/2, 5/2, etc.). Examples of fermions
are the protons and neutrons that make up an atom’s nucleus, and
electrons.
Composite particles, such as nuclei and atoms, are classified as
bosons or fermions based on the sum of the spins of their
constituent particles. For instance, an isotope of helium called
helium-4 turns out to be a bose particle. Helium-4 is made up of six
fermi particles: two electrons orbiting a nucleus made up of two
protons and two neutrons. Adding up six odd half-integers will yield
a whole integer, making helium-4 a boson. The atoms of rubidium used
in the Colorado experiment are bose particles as well. Only bose
atoms may form a condensate, but they do so only at a sufficiently
low temperature and high density.
At their lab in Colorado, Cornell and Wieman cooled a rubidium gas
down to a temperature as close to absolute zero, the temperature at
which particles stop moving, as they could get. The slower the
particles, the lower their momentum. In essence, the cooling brought
the momentum of the gas particles closer and closer to precisely
zero, as the temperature decreased to within a few billionths of a
degree Kelvin. (Kelvin degrees are on the scale of degrees Celsius,
but zero Kelvin is absolute zero, while zero Celsius is the freezing
point of water.)
As the temperature, and thus the momentum, of the gas particles
dropped to an infinitesimal amount, the possible locations of the
atom at any given moment increased proportionally. The goal of the
experiment was to keep the gas atoms packed together closely enough
that during this process—as their momentum got lower and lower, and
their wavelengths got larger and larger—their waves would begin to
overlap. This interplay of position and movement in three dimensions
with the relative distances between particles is known as the
phase-space density and is the key factor in forming a condensate.
In essence, the momentum of the atoms would become so precisely
pinpointed (near zero) that their position would become less and
less certain and there would be a relatively large amount of space
that would define each atom’s position. As the atoms slowed to
almost a stop, their positions became so fuzzy that each atom came
to occupy the same position as every other atom, losing their
individual identity. This odd phenomenon is a Bose-Einstein
condensate.
As their experimental conditions neared the realm of Bose-Einstein
condensation, Cornell and Wieman noticed an abrupt rise in the peak
density of their sample, a type of discontinuity that strongly
indicates a phase transition. The Colorado physicists estimated that
after progressive evaporative cooling of the rubidium, they were
left with a nugget of about 2,000 atoms of pure condensate.
Cornell
and Wieman then released the atoms from the "trap" in which they had
been cooling and sent a pulse of laser light at the condensate,
basically blowing it apart. They recorded an image of the expanding
cloud of atoms. Prior to the light pulse, when the density dropped
after the atoms were released, the physicists believed the
temperature of the condensate fell to an amazing frigidity of 20
nanoKelvins (20 billionths of one degree above absolute zero).
The image showed a larger, expanding sphere of particles with a
smaller, more concentrated elliptical-looking center. Cornell and
Wieman observed that when a gas is constrained and then released (in
an extreme example, as in a bomb), thermodynamics specifies that it
will expand outward equally in all directions regardless of the
shape in which it had been contained. This occurs because the
particles in that gas, even if the gas was very cold, were moving in
all different directions with various energies when the gas was
pushed outwards.
This rule of uniform expansion does not hold for a Bose-Einstein
condensate. Because the particles were all acting in exactly the
same manner at the time of the light pulse, their expansion should
give some indication of the shape of the space they had previously
inhabited. The uneven, elliptical-looking clump of atoms in the
center of the image recorded by Cornell and Wieman thus gave further
proof that a condensate had formed.
Bose-Einstein characteristics have been observed in other systems,
specifically, in superfluid liquid helium-4 and in superconductors.
It is believed that liquid helium-4 at a sufficiently low
temperature is composed of two components mixed together, the colder
of which is a Bose-Einstein condensate. Liquid helium-4, which at
very low temperatures is also a superconductor of heat, behaves in
dramatic ways, trickling up the sides of containers and rising in
fountains.
Electrical superconductors are also boson-related phenomena. In
superconductors, which are also formed by supercooling, electrical
resistance disappears. In this case it is the electrons within a
substance’s atoms, rather than the atoms themselves, that condense.
The electrons pair up, together forming a particle of zero spin.
These paired electrons merge into an overall substance that flows
freely through the superconductor, offering no resistance to
electric current.
Thus, once initiated, a current can flow
indefinitely in a superconductor.
Go Back
Quantum results
Quantum mechanics solved all of the great difficulties that troubled
physicists in the early years of the 20th century. It gradually
enhanced the understanding of the structure of matter, and it
provided a theoretical basis for the understanding of atomic
structure (see Atom and Atomic Theory) and the phenomenon of
spectral lines: Each spectral line corresponds to the energy of a
photon transmitted or absorbed when an electron makes a transition
from one energy level to another.
The understanding of chemical
bonding was fundamentally transformed by quantum mechanics and came
to be based on Schrödinger’s wave equations. New fields in physics
emerged—condensed matter physics, superconductivity, nuclear
physics, and elementary particle physics (see Physics)—that all
found a consistent basis in quantum mechanics.
Go Back
Developments
FURTHER DEVELOPMENTS: In the years since 1925, no fundamental
deficiencies have been found in quantum mechanics, although the
question of whether the theory should be accepted as complete has
come under discussion. In the 1930s the application of quantum
mechanics and special relativity to the theory of the electron (see
Quantum Electrodynamics) allowed the British physicist Paul Dirac to
formulate an equation that referred to the existence of the spin of
the electron. It further led to the prediction of the existence of
the positron, which was experimentally verified by the American
physicist Carl David Anderson.
The application of quantum mechanics to the subject of
electromagnetic radiation led to explanations of many phenomena,
such as bremsstrahlung (German, "braking radiation," the radiation
emitted by electrons slowed down in matter) and pair production (the
formation of a positron and an electron when electromagnetic energy
interacts with matter). It also led to a grave problem, however,
called the divergence difficulty: Certain parameters, such as the
so-called bare mass and bare charge of electrons, appear to be
infinite in Dirac’s equations.
(The terms bare mass and bare charge
refer to hypothetical electrons that do not interact with any matter
or radiation; in reality, electrons interact with their own electric
field.)
This difficulty was partly resolved in 1947-49 in a program
called renormalization, developed by the Japanese physicist
Shin’ichirô Tomonaga, the American physicists Julian S. Schwinger
and Richard Feynman, and the British physicist Freeman Dyson. In
this program, the bare mass and charge of the electron are chosen to
be infinite in such a way that other infinite physical quantities
are canceled out in the equations.
Renormalization greatly increased
the accuracy with which the structure of atoms could be calculated
from first principles.
Go Back
Electrons
Theoretical physicist C. Llewellyn Smith discusses the discoveries
that scientists have made to date about the electron and other
elementary particles—subatomic particles that scientists believe
cannot be split into smaller units of matter. Scientists have
discovered what Smith refers to as sibling and cousin particles to
the electron, but much about the nature of these particles is still
a mystery.
One way scientists learn about these particles is to
accelerate them to high energies, smash them together, and then
study what happens when they collide. By observing the behavior of
these particles, scientists hope to learn more about the fundamental
structures of the universe.
Electrons: The First Hundred Years
The discovery of the electron was announced by J. J. Thomson just
over 100 years ago, on April 30, 1897. In the intervening years we
have come to understand the mechanics that describe the behavior of
electrons—and indeed of all matter on a small scale—which is called
quantum mechanics. By exploiting this knowledge, we have learned to
manipulate electrons and make devices of a tremendous practical and
economic importance, such as transistors and lasers.
Meanwhile, what have we learned of the nature of the electron
itself? From the start, electrons were found to behave as elementary
particles, and this is still the case today. We know that if the
electron has any structure, it is on a scale of less than 1018 m,
i.e. less than 1 billionth of 1 billionth of a meter.
However, a major complication has emerged. We have discovered that
the electron has a sibling and cousins that are apparently equally
fundamental. The sibling is an electrically neutral particle, called
the neutrino, which is much lighter than the electron. The cousins
are two electrically charged particles, called the mu and the
tau,
which also have neutral siblings. The mu and the tau seem to be
identical copies of the electron, except that they are respectively
200 and 3,500 times heavier. Their role in the scheme of things and
the origin of their different masses remain mysteries — just the sort
of mysteries that particle physicists, who study the constituents of
matter and the forces that control their behavior, wish to resolve.
We therefore know of six seemingly fundamental particles, the
electron, the mu, the tau and their neutral siblings, which—like the
electron—do not feel the nuclear force, and incidentally are known
generically as leptons.
What about the constituents of atomic nuclei, which of course do
feel the nuclear force? At first sight, nuclei are made of protons
and neutrons, but these particles turned out not to be elementary.
It was found that when protons and neutrons are smashed together,
new particles are created. We now know that all these particles are
made of more elementary entities, called quarks. In a collision,
pairs of quarks and their antiparticles, called antiquarks, can be
created: part of the energy (e) of the incoming particles is turned
into mass (m) of these new particles, thanks to the famous
equivalence e = mc2. The quarks in the projectiles and the created
quark-antiquark pairs can then rearrange themselves to make various
different sorts of new particles.
Today, six types of quarks are known which, like the leptons (the
electron and its relations) have simple properties, and could be
elementary. In the past 30 years a recipe that describes the
behavior of these particles has been developed. It is called the
"Standard Model" of particle physics. However, we lack a real
understanding of the nature of these particles, and the logic behind
the Standard Model. What is wrong with the Standard Model?
First, it does not consistently combine Einstein’s theory of the
properties of space (called General Relativity) with a quantum
mechanical description of the properties of matter. It is therefore
logically incomplete.
Second, it contains too many apparently arbitrary futures—it is too
baroque, too byzantine—to be complete. It does not explain the role
of the mu and the tau, or answer the question whether the fact that
the numbers of leptons and quarks are the same—six each—is a
coincidence, or an indication of a deep connection between these
different types of particles. On paper, we can construct theories
that give better answers and explanations, and in which there are
such connections, but we do not know which, if any, of these
theories is correct.
Third, it has a missing, untested, element. This is not some minor
detail, but a central element, namely a mechanism to generate the
observed masses of the known particles, and hence also the different
ranges of the known forces (long range for gravity and
electromagnetism, as users of magnetic compasses know, but very
short range for the nuclear and the so-called weak forces, although
in every other respect these forces appear very similar). On paper,
a possible mechanism is known, called the Higgs mechanism, after the
British physicist Peter Higgs who invented it. But there are
alternative mechanisms, and in any case the Higgs mechanism is a
generic idea. We not only need to know if nature uses it, but if so,
how it is realized in detail.
Luckily the prospects of developing a deeper understanding are good.
The way forward is to perform experiments that can distinguish the
different possibilities. We know that the answer to the mystery of
the origin of mass, and the different ranges of forces, and certain
other very important questions, must lie in an energy range that
will be explored in experiments at the Large Hadron Collider, a new
accelerator now under construction at CERN [also known as the
European Laboratory for Particle Physics] near Geneva.
The fundamental tools on which experimental particle physics depends
are large accelerators, like the Large Hadron Collider, which
accelerate particles to very high energies and smash them together.
By studying what happens in the collisions of these particles, which
are typically electrons or protons (the nuclei of hydrogen atoms),
we can learn about their natures. The conditions that are created in
these collisions of particles existed just after the birth of the
universe, when it was extremely hot and dense. Knowledge derived
from experiments in particle physics is therefore essential input
for those who wish to understand the structure of the universe as a
whole, and how it evolved from an initial fireball into its present
form.
The Large Hadron Collider will therefore not only open up a large
new window on the nature of matter, when it comes into operation in
2005, but also advance our understanding of the structure of the
universe. However, although it will undoubtedly resolve some major
questions and greatly improve our knowledge of nature, it would be
very surprising if it established a "final theory."
The only candidate theory currently known which appears to have the
potential to resolve all the problems mentioned above—the reason for
the existence of the mu and tau, reconciliation of
Einstein’s
general relativity with quantum mechanics, etc.—describes the
electron and its relatives and the quarks, not as pointlike objects,
but as different vibrating modes of tiny strings. However, these
strings are so small (10-35 m) that they will never be observed
directly.
If this is so, the electron and the other known particles
will continue forever to appear to be fundamental pointlike objects,
even if the—currently very speculative—"string theory" scores enough
successes to convince us that this is not the case!
Go Back
The Future
FUTURE PROSPECTS: Quantum mechanics underlies current attempts to
account for the strong nuclear force and to develop a unified theory for all the fundamental interactions
of matter.
Nevertheless, doubts exist about the completeness of quantum theory.
The divergence difficulty, for example, is only partly resolved.
Just as Newtonian mechanics was eventually amended by quantum
mechanics and relativity, many scientists—and Einstein was among
them—are convinced that quantum theory will also undergo profound
changes in the future.
Great theoretical difficulties exist, for
example, between quantum mechanics and chaos theory, which began to
develop rapidly in the 1980s.
Ongoing efforts are being made by
theorists such as the British physicist Stephen Hawking, to develop
a system that encompasses both relativity and quantum mechanics.
Go Back
Quantum
Computers
Breakthroughs occurred in the area of quantum computing in the late
1990s. Quantum computers under development use components of a
chloroform molecule (a combination of chlorine and hydrogen atoms)
and a variation of a medical procedure called magnetic resonance
imaging (MRI) to compute at a molecular level. Scientists used a
branch of physics called quantum mechanics, which describes the
activity of subatomic particles (particles that make up atoms), as
the basis for quantum computing.
Quantum computers may one day be
thousands to millions of times faster than current computers,
because they take advantage of the laws that govern the behavior of
subatomic particles. These laws allow quantum computers to examine
all possible answers to a query at one time.
Future uses of quantum
computers could include code breaking and large database queries.
Go Back
Quantum Time Waits for No Cosmos
THE INTRIGUING notion that time might run backwards when the
Universe collapses has run into difficulties. Raymond Laflamme, of
the Los Alamos National Laboratory in New Mexico, has carried out a
new calculation which suggests that the Universe cannot start out
uniform, go through a cycle of expansion and collapse, and end up in
a uniform state. It could start out disordered, expand, and then
collapse back into disorder. But, since the COBE data show that our
Universe was born in a smooth and uniform state, this symmetric
possibility cannot be applied to the real Universe.
Physicists have long puzzled over the fact that two distinct "arrows
of time" both point in the same direction. In the everyday world,
things wear out -- cups fall from tables and break, but broken cups
never re- assemble themselves spontaneously. In the expanding
Universe at large, the future is the direction of time in which
galaxies are further apart.
Many years ago, Thomas Gold suggested that these two arrows might be
linked. That would mean that if and when the expansion of the
Universe were to reverse, then the everyday arrow of time would also
reverse, with broken cups re-assembling themselves.
More recently, these ideas have been extended into quantum physics.
There, the arrow of time is linked to the so-called "collapse of the
wave function", which happens, for example, when an electron wave
moving through a TV tube collapses into a point particle on the
screen of the TV. Some researchers have tried to make the quantum
description of reality symmetric in time, by including both the
original state of the system (the TV tube before the electron passes
through) and the final state (the TV tube after the electron has
passed through) in one mathematical description.
Murray Gell-Mann and James Hartle recently extended this idea to the
whole Universe. They argued that if, as many cosmologists believe
likely, the Universe was born in a Big Bang, will expand out for a
finite time and then recollapse into a Big Crunch, the time-neutral
quantum theory could describe time running backwards in the
contracting half of its life.
Unfortunately, Laflamme has now shown that this will not work. He
has proved that if there are only small inhomogeneities present in
the Big Bang, then they must get larger throughout the lifetime of
the Universe, in both the expanding and the contracting phases. "A
low entropy Universe at the Big Bang cannot come back to low entropy
at the Big Crunch" (Classical and Quantum Gravity, vol 10 p L79). He
has found time-asymmetric solutions to the equations -- but only if
both Big Bang and Big Crunch are highly disordered, with the
Universe more ordered in the middle of its life.
Observations of the cosmic microwave background radiation show that
the Universe emerged from the Big Bang in a very smooth and uniform
state. This rules out the time-symmetric solutions.
The implication
is that even if the present expansion of the Universe does reverse,
time will not run backwards and broken cups will not start re-
assembling themselves.
Go Back
|

 Quantum Theory, also quantum mechanics, in physics, a theory based
on using the concept of the quantum unit to describe the dynamic
properties of subatomic particles and the interactions of matter and
radiation. The foundation was laid by the German physicist Max
Planck, who postulated in 1900 that energy can be emitted or
absorbed by matter only in small, discrete units called quanta.
Quantum Theory, also quantum mechanics, in physics, a theory based
on using the concept of the quantum unit to describe the dynamic
properties of subatomic particles and the interactions of matter and
radiation. The foundation was laid by the German physicist Max
Planck, who postulated in 1900 that energy can be emitted or
absorbed by matter only in small, discrete units called quanta.